
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Install NowNCERT Solutions for Class 10 Science Acids, Bases and Salts Part 2 Class 10 Science Class book solutions are available in PDF format for free download. These ncert book chapter wise questions and answers are very helpful for CBSE Board exam. CBSE recommends NCERT books and most of the questions in CBSE exam are asked from NCERT text books. Class 10 Science chapter wise NCERT solution for Science Book for all the chapters can be downloaded from our website and myCBSEguide mobile app for free.
NCERT solutions for Class 10 Science Acids, Bases and Salts Download as PDF
NCERT Class 10 Science Chapter wise Solutions
- 1 – Chemical Reactions and Equations
- 2 – Acids, Bases and Salts
- 3 – Metals and Non-metals
- 4 – Carbon and Its Compounds
- 5 – Periodic Classification of Elements
- 6 – Life Processes
- 7 – Control and Coordination
- 8 – How do Organisms Reproduce?
- 9 – Heredity and Evolution
- 10 – Light Reflection and Refraction
- 11 – Human Eye and Colourful World
- 12 – Electricity
- 13 – Magnetic Effects of Electric Current
- 14 – Sources of Energy
- 15 – Our Environment
- 16 – Management of Natural Resources
NCERT Solutions for Class 10 Science Acids, Bases and Salts Part 2
1. A solution turns red litmus blue, its pH is likely to be
(a) 1
(b) 4
(c) 5
(d) 10
Ans. (d) 10
2. A solution reacts with crushed egg-shells to give a gas that turns lime-water milkey.
The solution contains
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
Ans. (b) HCl
3. 10 mL of a solution of NaOH is found to be completely neutralized by 8 mL of a given solution of HCl. If we take 20 mL of same solution of NaOH, the amount of HCl solution required to neutralize it will be
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Ans. (d) 16 mL
4. Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotics
(b) Analgesic
(c) Antacid
(d) Antiseptic
Ans. (c) Antacid
NCERT Solutions for Class 10 Science Acids, Bases and Salts Part 2
5. Write word equations and then balanced equations for the reaction taking place when:
(a) Dilute Sulphuric acid reacts with zinc granules.
(b) Dilute hydrochloric acid reacts with magnesium ribbon.
(c) Dilute Sulphuric acid reacts with aluminum powder
(d) Dilute hydrochloric acid reacts with iron fillings.
Ans. (a) Zinc + Sulphuric acid 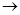 Zinc sulphate +Hydrogen
Zinc sulphate +Hydrogen
(b) Magnesium + Sulphuric acid 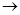 magnesium chloride +Hydrogen gas
magnesium chloride +Hydrogen gas
(c) Aluminum + Sulphuric acid 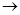 Aluminum sulphate +Hydrogen gas
Aluminum sulphate +Hydrogen gas
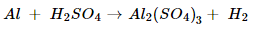
(d) Iron + Hydrochloric acid 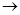 Iron chloride +Hydrogen
Iron chloride +Hydrogen
NCERT Solutions for Class 10 Science Acids, Bases and Salts Part 2
6. Compound such as alcohols and glucose also contain hydrogen but are not categorized as acids. Describe an activity.
Ans. Alcohol and glucose both contain hydrogen but not categorized as acids. This can be proved by following activity.
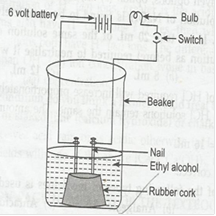
Material required: – Beaker, nails, battery, connecting wires, bulb, switch and alcohols.
Procedure:
1. Set up the experiment as follows
2. Take ethyl alcohol in the beaker in the beaker.
3. When the switch is turned on, the bulb does not glow.
4. Take glucose solution in place of alcohols but bulb does not glow.
7. Why does distilled water not conduct electricity, where as rain water does?
Ans. Rain water contains small amount of acid because of which it conducts electricity. Distilled water is pure water. It does not contain ions. Therefore, it does not conduct electricity.
8. Why do acids not show acidic behavior in the absence of water?
Ans. Acids produce hydrogen ions or hydronium ions only in presence of water. Therefore, it shows acidic behavior only presence of water.
NCERT Solutions for Class 10 Science Acids, Bases and Salts Part 2
9. Five solutions A, B, C, D and E when tested with universal indicators showed pH as 4, 1, 11, 7 and 9 respectively. Which solution is:
(a) neutral?
(b) strongly alkaline?
(c) strongly acidic
(d) weakly acidic?
(e) weakly alkaline
Ans. (a) D
(b) C
(c) B
(d) A
(e) E
NCERT Solutions for Class 10 Science Acids, Bases and Salts Part 2
10. Equal lengths of magnesium ribbons are taken in test tubes A and B. hydrochloric acid is added to test tube A, while acetic acid is added to test B. In which test tube will the fizzing occur more vigorously and why?
Ans. HCl is stronger acid than CH3COOH. Therefore, H+ ions concentration in test tube A will be more than that in test tube B. hence, reaction will take place faster in test tube A than in test tube B. so, fizzing will occur more vigorously in test tube B.
11. Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
Ans. Bacteria change the fresh milk into curd by producing lactic acid. Because of the presence of lactic acid in curd, the pH will come down from 6 to lower value.
NCERT Solutions for Class 10 Science Acids, Bases and Salts Part 2
12. A milkman adds a very small amount of baking soda to fresh milk.
(a) Why does he shift the pH of the milk from 6 to slightly alkaline?
(b) Why does this milk take a long time to set a curd?
Ans. (a) The pH of milk changes from 6 to slightly alkaline on addition of a very small amount of baking soda. This is because sodium hydrogen carbonate (baking soda) is basic in nature. This prevents the milk from souring.
(b) Lactic acid formed as a result of fermentation is neutralized by sodium hydrogen carbonate. This prolongs the time taken by milk to set as curd.
13. Plaster of Paris should be stored in moisture-proof container. Explain why?
Ans. Plaster of Paris reacts with moisture to form gypsum and sets to a hard mass. Therefore, it should be stored in moisture-proof container.
NCERT Solutions for Class 10 Science Acids, Bases and Salts Part 2
14. What is a neutralization reaction? Give two examples.
Ans. The reaction between an acid and a base to give salt and water is called neutralization reaction.
For example:
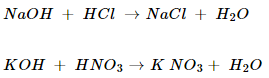
NCERT Solutions for Class 10 Science Acids, Bases and Salts Part 2
15. Give two important uses of washing soda and baking soda.
Ans. Uses of washing soda:
(i) As cleansing agent.
(ii) Removing permanent hardness of water.
(iii) Used in glass, soap and paper industries.
Uses of baking soda:
(i) For making baking powder.
(ii) As ingredient of antacid.
NCERT Solutions for Class 10 Science
NCERT Solutions Class 10 Science PDF (Download) Free from myCBSEguide app and myCBSEguide website. Ncert solution class 10 Science includes text book solutions from Book. NCERT Solutions for CBSE Class 10 Science have total 16 chapters. 10 Science NCERT Solutions in PDF for free Download on our website. Ncert Science class 10 solutions PDF and Science ncert class 10 PDF solutions with latest modifications and as per the latest CBSE syllabus are only available in myCBSEguide.
CBSE app for Students
To download NCERT Solutions for class 10 Social Science, Computer Science, Home Science,Hindi ,English, Maths Science do check myCBSEguide app or website. myCBSEguide provides sample papers with solution, test papers for chapter-wise practice, NCERT solutions, NCERT Exemplar solutions, quick revision notes for ready reference, CBSE guess papers and CBSE important question papers. Sample Paper all are made available through the best app for CBSE students and myCBSEguide website.

Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Create Now
myCBSEguide
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Install Now
Class 10 Science MCQ