
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Install NowCBSE Sample Papers Class 11 Chemistry (2023-24)
We at myCBSEguide provide CBSE class 11 Sample Papers of chemistry for the year 2024 with solutions in PDF format for free download. Students must download and practice the CBSE Sample Papers, and questions from NCERT books which are based on the latest syllabus issued by CBSE. Class 11 Chemistry New Sample Papers (2023-24) follow the blueprint of the current academic session only. All the students must check the latest syllabus and marking scheme of class 11th Chemistry. Sample papers for Class 11 Chemistry and other subjects are available for download as PDFs in the myCBSEguide app too. The myCBSEguide app has sample papers with solutions for the session 2023-24 on all major subjects across the streams.
Download Chemistry Sample Papers as PDF
Class 11 – Chemistry Sample Paper (2023-24)
Maximum Marks: 70
Time Allowed: : 3 hours
General Instructions:
- There are 33 questions in this question paper with internal choice.
- SECTION A consists of 16 multiple-choice questions carrying 1 mark each.
- SECTION B consists of 5 very short answer questions carrying 2 marks each.
- SECTION C consists of 7 short answer questions carrying 3 marks each.
- SECTION D consists of 2 case-based questions carrying 4 marks each.
- SECTION E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
- The use of log tables and calculators is not allowed.
Class 11 Chemistry Sample Paper Section A
- If the density of a solution is 3.12 g mL–1, the mass of 1.5 mL solution in significant figures is _______.a) 4680 × 10–3gb) 4.7gc) 47.80gd) 4.680g
- Oil drop experiment is for determining the:a) deviation of the electron.b) mass of the electronc) number of electronsd) charge on the electrons
- The enthalpies of elements in their standard states are taken as zero. The enthalpy of formation of a compound is:a) is never negative.b) may be positive or negative.c) is always negative.d) is always positive.
- If nucleon (mass) and proton (atomic) number is 40 and 20 respectively, element isa) chlorineb) phosphorusc) potassiumd) calcium
- Enthalpies of formation of CO(g), CO2(g), N2O(g), and N2O4(g) are -110, –393, 81, and 9.7 kJ mol-1 respectively. Find the value of {tex}{\Delta _{\text{r}}}{\text{H}}{/tex} for the reaction:{tex}{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}\left( {\text{g}} \right){\text{ }} + {\text{ 3CO}}\left( {\text{g}} \right){\text{ }} \to {\text{ }}{{\text{N}}_{\text{2}}}{\text{O}}\left( {\text{g}} \right){\text{ }} + {\text{ 3C}}{{\text{O}}_{\text{2}}}\left( {\text{g}} \right){/tex}a) – 850 kJb) -600 kJc) -778 kJd) -802 kJ
- de-Broglie equation isa) {tex}\lambda{/tex} = {tex}\frac{hv}{m}{/tex}b) {tex}\lambda{/tex} = {tex}\frac{mv}{h}{/tex}c) {tex}\lambda{/tex} = hmvd) {tex}\lambda{/tex} = {tex}\frac{h}{m v}{/tex}
To practice more questions & prepare well for exams, download myCBSEguide App. It provides complete study material for CBSE, NCERT, JEE (main), NEET-UG and NDA exams. Teachers can use Examin8 App to create similar papers with their own name and logo.
- In which of the following compounds, an element exhibits two different oxidation states.a) NH4NO3b) N3Hc) N2H4d) NH2OH
- Which of the following carbocation is most stable?a) {tex}{(C{H_3})_3}\mathop C\limits^ \oplus {/tex}b) {tex}{(C{H_3})_3}C\mathop C\limits^ \oplus {H_2}{/tex}c) {tex}C{H_3}\mathop C\limits^ \oplus HC{H_2}C{H_3}{/tex}d) {tex}C{H_3}C{H_2}\mathop C\limits^ \oplus {H_2}{/tex}
- Baeyer’s reagent is:a) K2MnO4b) alkaline KMnO4c) bromine waterd) acidified KMnO4
- Which of the following compounds is/are amphoteric in nature?a) As2O3b) Both AI2O3 and As2O3c) CI2O7d) AI2O3
- Which of the following conditions is/are applied for the measurement made in calorimeter?a) Both Constant volume, qv or Constant pressure, qpb) Constant temperature, qTc) Constant pressure, qpd) Constant volume, qv
- An aqeous solution of compound A gives ethane on electrolysis. The compound A is …..?a) Sodium propionateb) Sodium acetatec) Sodium ethoxided) Ethyl acetate
- Assertion (A): The resonance structure is hypothetical and individually represents any real molecules.
Reason (R): According to the resonance theory the actual structure of benzene cannot be adequately represented by any of these structures.a) Both A and R are true and R is the correct explanation of A.b) Both A and R are true but R is not the correct explanation of A.c) A is true but R is false.d) A is false but R is true. - Assertion (A): Addition of HBr on
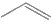 in presence of peroxide give
in presence of peroxide give  as major product.
as major product.
Reason (R): Addition of HBr on alkene proceed by carbocation intermediate.a) Both A and R are true and R is the correct explanation of A.b) Both A and R are true but R is not the correct explanation of A.c) A is true but R is false.d) A is false but R is true. - Assertion (A): All isotopes of a given element show the same type of chemical behaviour.
Reason (R): The chemical properties of an atom are controlled by the number of electrons in the atom.a) Both A and R are true and R is the correct explanation of A.b) Both A and R are true but R is not the correct explanation of A.c) A is true but R is false.d) A is false but R is true. - Assertion (A): Both 44g CO2 and 16g CH4 have same number of carbon atoms.
Reason (R): Both contain 1 g atom of carbon which contains 6.023 {tex}\times{/tex} 1023 carbon atoms.a) Both A and R are true and R is the correct explanation of A.b) Both A and R are true but R is not the correct explanation of A.c) A is true but R is false.d) A is false but R is true. Class 11 Chemistry Sample Paper Section B
- Calculate the molar solubility of Ni(OH)2 in 0.10M NaOH. The ionic product of Ni(OH)2 is 2.0 {tex}\times{/tex} 10-15.
- What do you understand by exothermic reaction and endothermic reaction? Give one example of each type.
- Calculate: Number of gram atoms in 1.4 grams of nitrogen. (Atomic mass, N = 14)
- Draw the structure of the following compounds all showing C and H atoms.
- 2-methyl -3-isopropyl heptane
- Dicyclopropyl methane.
OR
Write equations for the preparation of
- HC{tex}\equiv{/tex}CD and
- DC{tex}\equiv{/tex}CD
- What is the mass (me) of an electron?
Class 11 Chemistry Sample Paper Section C
- Explain with the help of suitable example polar covalent bond.
- Answer the following:
- Two liters of an ideal gas at a pressure of 10 atm expands isothermally at 25 °C into a vacuum until its total volume is 10 liters. How much heat is absorbed and how much work is done in the expansion?
- Identify the state functions and path functions out of the following.
Enthalpy, entropy, heat, temperature, work, free energy. - If enthalpy of fusion and enthalpy of vaporisation of sodium metals are 2.6 and 98.2 kJ mol-1 respectively, what is the enthalpy of sublimation of sodium?
- Calculate the standard Gibbs energy change for the formation of propane at 298 K.
3C (graphite) + 4H2(g) {tex}\to{/tex} C3H8(g)
{tex}\Delta_fH^o{/tex} for propane, C3H8(g) is – 103.8 kJ mol-1
Given, {tex}s_m^{\circ}{/tex} C3H8(g) = 270.2 JK-1mol-1
{tex}s_m^{\circ} C{/tex} (graphite) = 5.70 JK-1mol-1
and {tex}s_m^{\circ}{/tex}H2(g) = 130.7JK-1mol-1 - In the reactions given below, identify the species undergoing oxidation and reduction.
- H2S(g) + CI2(g) {tex}\rightarrow{/tex} 2HCl(g) + S(s)
- 3Fe3O4(s) + 8 AI(s) {tex}\rightarrow{/tex} 9 Fe(s) + 4Al2O3(s)
- 2Na (s) + H2(g) {tex}\rightarrow{/tex} 2NaH(s)
- Calculate the energy required for the process:
He+ (g) {tex}\longrightarrow{/tex} He2+ (g) + e–
The ionization energy for the H atom in the ground state is 2.18 {tex}\times{/tex} 10-18 J atom. - An element ‘X’ with atomic number 112 has been recently predicted. Its electronic configuration is : [Rn] 5f146d107s2 . Predict
- its group
- block in which this element would be placed
- IUPAC name and symbol.
- Commercially available concentrated hydrochloric acid(HCl) contains 38% HCI by mass.
- What is the molarity (M) of the solution (density of solution = 1.19 g mL-1)
- What volume required of concentrated HCI is required to make 1.0 L of an 0.10 M HCI?
Class 11 Chemistry Sample Paper Section D
- Read the text carefully and answer the questions:
IUPAC (International Union of Pure and Applied Chemistry) system of nomenclature. Common names are useful and in many cases indispensable, particularly when the alternative systematic names are lengthy and complicated. A systematic name of an organic compound is generally derived by identifying the parent hydrocarbon and the functional group(s) attached to it. By using prefixes and suffixes, the parent name can be modified to obtain the actual name. In a branched-chain compound, small chains of carbon atoms are attached at one or more carbon atoms of the parent chain. The small carbon chains (branches) are called alkyl groups. An alkyl group is derived from a saturated hydrocarbon by removing a hydrogen atom from carbon. Abbreviations are used for some alkyl groups. For example, methyl is abbreviated as Me, ethyl as Et, propyl as Pr and butyl as Bu.- Draw the structure of 3-Ethyl-4,4-dimethylheptane.
OR
Why CH4 after becoming-CH3 called a methyl group?
- How is the numbering in branched chain hydrocarbon done?
- Derive the structure of 2-Chlorohexane.
- Read the text carefully and answer the questions:
When anions and cations approach each other, the valence shell of anions are pulled towards the cation nucleus and thus, the shape of the anion is deformed. The phenomenon of deformation of anion by a cation is known as polarization and the ability of the cation to polarize the anion is called as polarizing power of cation. Due to polarization, sharing of electrons occurs between two ions to some extent and the bond shows some covalent character.
The magnitude of polarization depends upon a number of factors.- Out of AlCl3 and AlI3 which halides show maximum polarization?
- Out of AlCl3 and CaCl2 which one is more covalent in nature?
- The non-aqueous solvent like ether is added to the mixture of LiCl, NaCl and KCl. Which will be extracted into the ether?
OR
Out of CaF2 and CaI2 which one has a minimum melting point?
To practice more questions & prepare well for exams, download myCBSEguide App. It provides complete study material for CBSE, NCERT, JEE (main), NEET-UG and NDA exams. Teachers can use Examin8 App to create similar papers with their own name and logo.
Class 11 Chemistry Sample Paper Section E
- Attempt any five of the following:
- What happens to equilibrium constant when temperature increases for a reaction?
- Explain why alkynes are less reactive than alkenes towards addition of Br2.
- What is a Lindlars’ catalyst?
- Why do alkynes not show geometrical isomerism?
- Which conformation of ethane is more stable?
- State Le chatelier’s principle.
- How is alkene produced by vicinal dihalide?
- Write a relation between {tex}\triangle G{/tex} and Q and define the meaning of each term and answer the following:
- Why a reaction proceeds forward when Q < K and no net reaction occurs when Q = K.
- Explain the effect of an increase in pressure in terms of reaction quotient Q for the reaction:
CO(g) + 3H2(g) {tex} \rightleftharpoons {/tex} CH4(g) + H2O(g)
OR
Ethyl acetate is formed by the reaction of ethanol and acetic acid and the equilibrium is represented as:
CH3COOH(l) + C5H5OH(l) {tex}\rightleftharpoons{/tex} CH3COOC2H5(l) + H2O(l)- Write the concentration ratio (reaction quotient), Qc, for this reaction (note: water is not in excess and is not a solvent in this reaction)
- At 293 K, if one starts with 1.00 mol of acetic acid and 0.18 mol of ethanol, there is 0.171 mol of ethyl acetate in the final equilibrium mixture. Calculate the equilibrium constant.
- Starting with 0.5 mol of ethanol and 1.0 mol of acetic acid and maintaining it at 293 K, 0.214 mol of ethyl acetate is found after some time. Has equilibrium been reached?
- Answer the following:
- Give the IUPAC name of the following compound
{tex}{\text{C}}{{\text{H}}_3} – \mathop {\mathop {{\text{CH}}}\limits_| }\limits_{{\text{Br}}} – \mathop {\mathop {\text{C}}\limits_{||} }\limits_{\text{O}} – \mathop {\mathop {{\text{CH}}}\limits_| }\limits_{{\text{C}}{{\text{H}}_{\text{3}}}} – {\text{C}}{{\text{H}}_3}{/tex} - 0.12 g of an organic compound containing phosphorous gave 0.22 g of Mg2P2O7 by usual analysis. Calculate the percentage of phosphorous in the compound.
OR- Which of the two structures CH3COOH and CH3COO– is more stabilized by resonance? Explain.
- Will CCl4 give a white precipitate of AgCl on heating it with AgNO3?
- Give the IUPAC name of the following compound
Class 11 – Chemistry Sample Paper Solution (2023-24)
Class 11 Chemistry Sample Paper Solution Section A
- (b) 4.7g
Explanation: Mass = Density {tex}\times{/tex} Volume
= 3.12 g mL-1 {tex}\times{/tex} 1.5 mL = 4.68 g = 4.7 g
significant figures as that of the least given number. Therefore, correct answer is 4.7 g - (d) charge on the electrons
Explanation: The oil drop experiment was performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron). The experiment entailed observing tiny electrically charged droplets of oil located between two parallel metal surfaces, forming the plates of a capacitor. - (b) may be positive or negative.
Explanation: Standard molar enthalpy of formation of a compound from its elements can be +ve or –ve.
For example: {tex}C+O_{2}(g) \rightarrow C O_{2}(g) ; \Delta_{r} H{/tex} = 393.5 kJ mol-1
{tex}N_{2}(g)+\frac{1}{2} O_{2}(g) \rightarrow N_{2} O(g);{/tex} {tex}\Delta_{r} H{/tex} = +92 k J mol-1 - (d) calcium
Explanation: calcium - (c) -778 kJ
Explanation: Heat of reaction, {tex}\Delta_r{/tex}H = {tex}{\textstyle\sum_{}}\triangle_rH_{products}-{\textstyle\sum_{}}{\textstyle{\scriptstyle\triangle}_r}{\textstyle{\scriptstyle H}_{reactants}}{\textstyle\;}{\textstyle\;}{/tex}
{tex}\Rightarrow{/tex} {tex}\Delta_{r} H{/tex} = [{tex}\Delta_{f} H{/tex}(N2O) + 3{tex}\Delta_{f} H{/tex}(CO2)] – [{tex}\Delta_{f} H{/tex}(N2O4) + 3{tex}\Delta_{f} H{/tex}(CO)] {tex}\Rightarrow{/tex} {tex}\Delta_{r} H{/tex} = [81 + {3 {tex}\times{/tex} (-393)}] – 99.7 + {3 {tex}\times{/tex} (-110)}] kJ
{tex}\Rightarrow{/tex} {tex}\Delta_{r} H{/tex} = -777.7 kJ {tex}\approx{/tex} -778 kJ
To practice more questions & prepare well for exams, download myCBSEguide App. It provides complete study material for CBSE, NCERT, JEE (main), NEET-UG and NDA exams. Teachers can use Examin8 App to create similar papers with their own name and logo. - (d) {tex}\lambda{/tex} = {tex}\frac{h}{m v}{/tex}
Explanation: Louis de-Broglie proposed that matter, like light , has a dual character.It exhibits wave as well as particle nature. The wavelength of the wave associated with a particle of mass m moving with velocity v is given by
{tex}\lambda{/tex} = {tex}\frac{h}{m v}{/tex} - (a) NH4NO3
Explanation: NH4+NO3–.In NH4+
N is -3 and
NO3– , N is +5. - (a) {tex}{(C{H_3})_3}\mathop C\limits^ \oplus {/tex}
Explanation: {tex}{(C{H_3})_3}\mathop C\limits^ \oplus {/tex} i.e. the tertiary carbocation is most stable. Alkyl groups directly attached to the positively charged carbon stabilise the carbocations due to inductive and hyperconjugation effects. - (b) alkaline KMnO4
Explanation: Baeyer’s reagent is a dilute alkaline KMnO4 solution. It is violet in colour when this solution reacts with the double bond the colour diaper and the whole solution become colourless. - (b) Both AI2O3 and As2O3
Explanation: A12O3 and As2O3 are amphoteric in nature. Amphoteric oxides behave as acidic with bases and basic with acids. - (a) Both Constant volume, qv or Constant pressure, qp
Explanation: In calorimetry, vessel called calorimeter is immersed in a known volume of a liquid. Measurements are made under two different conditions.- At constant volume, qv
- At constant pressure, qp
- (b) Sodium acetate
Explanation: This is an example of Kolbe’s electrolysis method. The reaction is:
{tex} { 2CH_3COONa + 2H_2O } \overset{_{electrolysis}}\longrightarrow{ CH_3.CH_3 + 2NaOH + H_2 + 2CO_2}{/tex}
The step-wise redox reactions occuring in the electrolytic cell are depicted as under
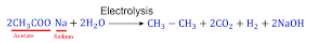
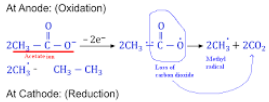
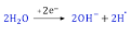
2H– {tex}\rightarrow{/tex} H2 - (d) A is false but R is true.
Explanation: The resonance structure is hypothetical and individually does not represents any real molecules. According to the resonance theory, the actual structure of benzene cannot be adequately represented by any of these structures. - (b) Both A and R are true but R is not the correct explanation of A.
Explanation: Both A and R are true but R is not the correct explanation of A. - (a) Both A and R are true and R is the correct explanation of A.
Explanation: All isotopes have the same atomic number means the same number of electrons and they bear similar chemical properties. - (a) Both A and R are true and R is the correct explanation of A.
Explanation: 44g of CO2 = 1 mole {tex}\equiv{/tex} 1 g atom of C
16g CH4 = 1 mole {tex}\equiv{/tex} 1 g atom of C
1 g atom of C = 12 g C
12 g C contains 6.023 {tex}\times{/tex} 1023 carbon atoms. Class 11 Chemistry Sample Paper Solution Section B
- According to the question, ionic product of Ni(OH)2 is 2.0 × 10-15.
Reaction:
Ni(OH)2 {tex}\rightleftharpoons{/tex} Ni2+ + 2OH–
NaOH {tex}\rightarrow{/tex} Na+ + OH–
Here, [OH–]total = (2S + 0.10)
So, Ksp = [Ni2+] [OH–]2
{tex}\Rightarrow{/tex}2.0 {tex}\times{/tex} 10-15 = (S) (0.10 +2S)2
As Ksp is small, 2S << 0.10,
{tex}\Rightarrow{/tex} 2.0 {tex}\times{/tex} 10-15 = S(0.10)2
{tex}\therefore{/tex} [Ni2+] = S = 2.0 {tex}\times{/tex} 10-13 M. - Exothermic reaction : The reaction accompanied by release of energy in the form of light or heat is called exothermic reaction. {tex}\Delta H{/tex} is negative for exothermic reaction.
Example: C+ O2 → CO2 ; {tex}\Delta H{/tex}= -178.3 kJmol-1.
Endothermic reactions : The reaction accompanied by absorption of energy is called endothermic reaction. {tex}\Delta H{/tex} is positive for endothermic reaction.
Example: 2NH3 → N2 +3H2. {tex}\Delta H{/tex} = +91.8kJmol-1. - Since, 1 gram atom N
= atomic mass of nitrogen (N) in grams.
= 14g
{tex}\therefore{/tex} 1.4 g of N
[{tex}\frac{1}{14} \times 1.4{/tex} ]gram atoms
= 0.1 gram atoms- The struture of 2-methyl -3-isopropyl heptane is:
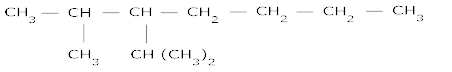
- The struture of Dicyclopropyl methane is:
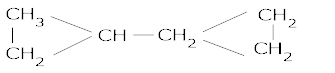
- The struture of 2-methyl -3-isopropyl heptane is:
OR
- {tex}\mathrm{HC}\equiv\mathrm{CH} \stackrel{\mathrm{NaNH}_{2}}{\longrightarrow} \mathrm{HC} \equiv \mathrm{C}^{-} \mathrm{Na}^{+} \stackrel{\mathrm{D}_{2} \mathrm{O}}{\longrightarrow} \mathrm{HC}\equiv\mathrm{CD}{/tex}
Here, Strong base NaNH2 deprotonated the alkynes to give so-called acetylide ions. - {tex}\mathrm{HC} \equiv \mathrm{CH} \stackrel{2 \mathrm{Na}}{\longrightarrow} \mathrm{Na}^{+} \mathrm{C}^{-}\equiv\mathrm{C}^{-} \mathrm{Na}^{+} \stackrel{\mathrm{D}_{2} \mathrm{O}}{\longrightarrow} \mathrm{DC} \equiv \mathrm{CD}{/tex}
Here, sodium metal is one-electron reducing agent.
- {tex}\mathrm{HC}\equiv\mathrm{CH} \stackrel{\mathrm{NaNH}_{2}}{\longrightarrow} \mathrm{HC} \equiv \mathrm{C}^{-} \mathrm{Na}^{+} \stackrel{\mathrm{D}_{2} \mathrm{O}}{\longrightarrow} \mathrm{HC}\equiv\mathrm{CD}{/tex}
- Mass of an electron (me) = {tex}\frac{Charge\;on\;electron}{Charge\;to\;Mass\;ratio}{/tex} = {tex}\frac { e } { \frac em }{/tex} {tex}= \frac { 1.602 \times 10 ^ { – 19 } C } { 1.76 \times 10 ^ { 8 } C g ^ { – 1 } }{/tex}{tex}= 9.10 \times 10 ^ { – 28 } g{/tex} {tex}= 9.1 \times 10 ^ { – 31 } k g{/tex}
Therefore, the mass of an electron is {tex}9.1 \times 10 ^ { – 31 } k g{/tex}.
Mass of an electron ={tex}\frac{1 }{1837}{/tex} th of the mass of a hydrogen atom. Class 11 Chemistry Sample Paper Solution Section C
- When two atoms with different electro-negativity are linked to each other by a covalent bond, the shared pair of electrons will not in the center because of the difference in electro-negativity. For example, in hydrogen fluoride.
molecule, fluorine has greater electronegativity than hydrogen. Thus the shared pair of electrons is displaced more towards fluorine atom, and acquire a partial negative charge ({tex}\delta^-{/tex}). At the same time, the hydrogen atom will have a partial positive charge ({tex}\delta^+{/tex}). Such a covalent bond is known as polar covalent bond or simply polar bond.
lt is represented as:
{tex}\delta^+{/tex} {tex}\delta^-{/tex}
H – F
(2,1) (4,0) - Answer:
- We have q = – w = pex (10 – 2) = 0(8) = 0 No work is done; no heat is absorbed.
- Path function: heat, work
State function: enthalpy, entropy, temperature, free energy. - According to the question, enthalpy of fusion and enthalpy of vaporisation of sodium metal is 2.6 kJ mol-1 and 98.2 kJ mol-1.
We know that, enthalpy of sublimation = {tex}\Delta _ { \mathrm { sub } } H ^ { \circ } = \Delta _ { \mathrm { fus } } H ^ { \circ } + \Delta _ { \mathrm { vap } } H ^ { \circ }{/tex}
= 2.6 + 98.2
= 100.8 kJ mol-1
- 3C(graphite) + 4H2(g) {tex}\to{/tex} C3H8(g)
{tex}\Delta_rS{/tex} = {tex}\Sigma S_m^{\circ}{/tex} (products) – {tex}S_m^{\circ}{/tex} (reactants)
{tex}\Delta_rS{/tex} = {tex}S_m^{\circ}{/tex} [C3H8(g) – 3{tex}S_m^{\circ}{/tex} [C (graphite)] + 4{tex}S_m^{\circ}{/tex}m[H2]] = (270.2 – 3 {tex}\times{/tex} 5.70 – 4 {tex}\times{/tex} 130.7) JK-1mol-1
= (270.2 – 17.10 – 522.80) JK-1mol-1
= (270.2 – 539.90) = – 269.7 JK-1mol-1
{tex}\Delta_rH^o{/tex} = {tex}\Delta_fH^o{/tex} products – {tex}\Delta_fH^o{/tex} reactants
{tex}\Delta_rH^o{/tex} = {tex}\Delta_fH^o{/tex} (C3H8) – 4{tex}\Delta_fH^o{/tex} [H2(g)] – 3{tex}\Delta_fH^o{/tex} [C (graphite)] {tex}\Delta_rH^o{/tex} = – 103.8 kJ mol-1; {tex}\Delta_rG^o{/tex} = {tex}\Delta_rH^o{/tex} – {tex}T\Delta_rS^o{/tex}
{tex}=\left(-103.8-\frac{298 \times(-269.7)}{1000}\right) \mathrm{kJ} \mathrm{mol}^{-1}{/tex}
= (-103.80 + 80.370) kJmol-1
= – 23.43 kJ mol-1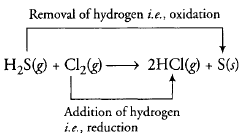
Thus, here, H2S undergoes oxidation because oxidation number of Sulphur atom get increased from -2 to 0 while Cl2 undergoes reduction because oxidation number of Cl atom get decreased from 0 to -1.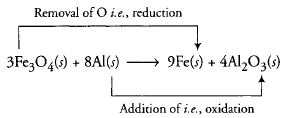
Thus, here Fe3O4 undergoes reduction because oxidation number of Fe atom get decreased while AI atom undergoes oxidation because Oxidation number of Al atom get increased from 0 to +3.- 2 Na(s) + H2(g) {tex}\rightarrow{/tex} 2 NaH(s)
Since Na is more electro-positive than H so, here Na atom get oxidised by losing electron while H-atom get reduced by accepting electron.
- The expression for the ionization energy atom:
En = {tex}\frac{2.18 \times 10^{-18} \times Z^{2}}{\mathrm{n}^{2}}{/tex} J atom-1
For H atom (Z = 1) En = 2.18 {tex}\times{/tex}10-18 {tex}\times{/tex}(1)2 J atom-1 (given)
For He+ ion (Z = 2) En = 2.18 {tex}\times{/tex}10-18 (2)2 = 8.72 {tex}\times{/tex} 10-18 J atom-1
(one electron species) - The configuration of the element is:{tex}[\mathrm{Rn}] 5 f^{14} 6 d^{10} 7 s^{2}{/tex}
- It belongs to the 12th group.
- It belongs to d block.
- IUPAC name is: Ununbium; Symbol: Uub.
- Let assume the total mass of the solution is 100g.
38 % HCI by mass means 38 g of HCI is present in 100 g of solution.
The volume of solution (V) = {tex}\frac { \text { mass } } { \text { density } } = \frac { 100 } { 1.19 } = 84.03 \mathrm { mL }{/tex} (Density of solution = 1.19 g/mL)
Number of moles of HCl (nB) = {tex}\frac{38}{36.5}{/tex} = 1.04
Molarity = {tex}\frac{n_B \times 1000}{V (\ in \ mL)}{/tex}= {tex}\frac{1.04 \times 1000}{84.03 \ mL}{/tex}= 12.38 M - From the molarity equation, {tex}\begin{array} { l } { M _ { 1 } V _ { 1 } = M _ { 2 } V _ { 2 } } \\ { \text { acid } _ { 1 } \quad \text { acid } _ { 2 } } \end{array}{/tex}
{tex}12.38 \mathrm { M } \times V _ { 1 } = 0.10 \mathrm { M } \times 1.0 \mathrm { L }{/tex}
{tex}\therefore{/tex} {tex}V _ { 1 } = \frac { 0.1 \times 1.0 } { 12.38 } = 0.00808 \mathrm { L } = 8.08 \mathrm { cm } ^ { 3 }{/tex}
Class 11 Chemistry Sample Paper Solution Section D
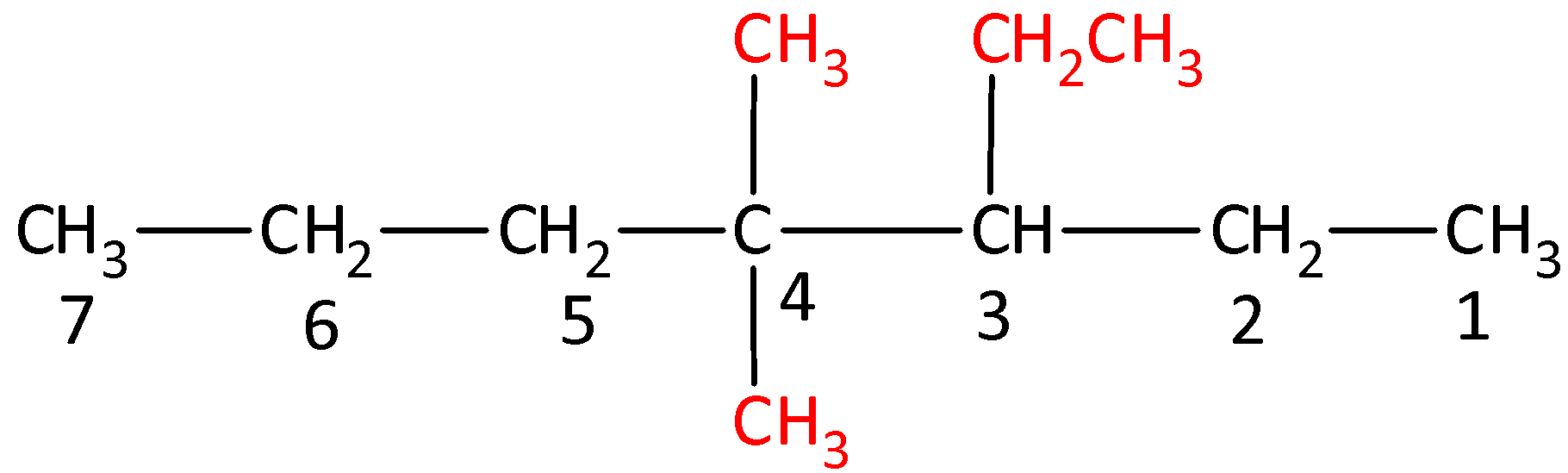
OR
CH4 after becoming-CH3 called a methyl group because an alkyl group is named by substituting ‘yl’ for ‘ane’ in the corresponding alkane.
- The numbering is done in such a way that the branched carbon atoms get the lowest possible numbers.
- ‘Hexane’ indicates the presence of 6 carbon atoms in the chain. The functional group chloro is present at carbon 2. Hence, the structure of the compound is CH2CH2CH2CH2CH(Cl)CH3.
- AlI3 halides show maximum polarization. The most covalent halide is AlI3.
Since lesser, the electronegativity difference, the more covalent is the aluminum halide. - AlCl3 is more covalent in nature.
- LiCl will be extracted into the ether.
OR
CaI2 has a minimum melting point.
- AlI3 halides show maximum polarization. The most covalent halide is AlI3.
Class 11 Chemistry Sample Paper Solution Section E
- Attempt any five of the following:
- Equilibrium constants gets changed if we change the temperature of the system.
- For Exothermic reactions, if the temperature is increased, the equilibrium will shift to favour the reaction which will decrease the temperature and the exothermic reaction is favoured.
- For Endothermic reactions, if the temperature is increased, the equilibrium will shift to favour the reaction which will reduce the temperature and the endothermic reaction is favoured.
- The triple bonds of alkynes, because of its high electron density, are easily attacked by electrophiles, but less reactive than alkenes due to the compact C-C electron cloud. The three-membered ring bromonium ion formed from the alkyne (A) has a full double bond causing it to be more stained and less stable than the one from the alkene (B),
Also, the carbon’s of (A) that are part of the bromonium ion has more s-character than (B), further making (A) less stable than (B). - Lindlar’s catalyst: Partially deactivated palladised charcoal is known as Lindlar’s catalyst.
Uses: Alkynes on partial reduction with calculated amount of dihydrogen in the presence of palladised charcoal partially deactivated with poisons like sulphur compounds or quinoline give alkenes. - Alkynes have a linear structure. Alkynes have triple bond. So, rotation is not possible. Hence, alkynes cannot show geometrical isomerism.
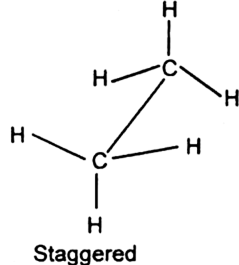
- Staggered conformation of ethane is more stable.
Structure:
- Le chatelier’s principle: If a system at equilibrium is subjected to change in the temperature, pressure or concentration of the reactants or the products that govern the equilibrium, then the equilibrium shifts in that direction in which this change is reduced or nullified.
- Alkene are produced from Vicinal dihalide by the process of dehalogenations. Vicinal dihalide on treatment with Zn metal lose a molecule of ZnX2 to from an alkene.
CH2Br-CH2Br + Zn → CH2=CH2 + ZnBr2.
To practice more questions & prepare well for exams, download myCBSEguide App. It provides complete study material for CBSE, NCERT, JEE (main), NEET-UG and NDA exams. Teachers can use Examin8 App to create similar papers with their own name and logo.
- We know that, {tex}\Delta G = \Delta G ^ { \circ } + R T \operatorname { ln } Q{/tex}
Where,
{tex} \Delta G ^ { \circ } {/tex} = Change in free energy as the reaction proceeds
{tex}\Delta G{/tex}= Standard free energy change
Q = Reaction quotient
T = Absolute temperature
Also, {tex} \Delta G ^ { \circ } = -RT\ lnK{/tex}
{tex}\Rightarrow \Delta G = – R T \ l n K + R T l n Q \\\therefore \Delta G= R T \ l n \frac { Q } { K }{/tex}
If Q < K, {tex}\Delta G{/tex} will be negative. So, the reaction proceeds in the forward direction.
If Q = K, {tex}\Delta G{/tex} = 0, reaction will be at equilibrium. - Reaction:
CO(g) + 3H2(g) {tex} \rightleftharpoons {/tex} CH4(g) + H2O(g)
On increasing the pressure equilibrium will shift in forward direction, it means Q < K.
- Equilibrium constants gets changed if we change the temperature of the system.
OR
- The concentration ratio (concentration quotient) Qc for the reaction is:
{tex}{Q_c} = \frac{{[C{H_3}COO{C_2}{H_5}(l)][{H_2}O(l)]}}{{[C{H_3}COOH(l)][{C_2}{H_2}OH(l)]}}{/tex} - {tex}\begin{array}{*{20}{c}} {}&{C{H_3}COOH(l) + }&{{C_2}{H_5}OH(l) \rightleftharpoons }&{C{H_3}COO{C_2}{H_2}(l) + } \\ {Initial\;molar\;conc.}&{1.0\;mol}&{0.18\;mol}&0 \\ {Molar\;conc.\;at}&{(1 – 0.171)}&{(0.18 – 0.171)}&{0.171\;mol} \\ {equilibrium\;po\operatorname{int} }&{ = 0.829\;mol}&{ = 0.009}&{} \end{array}\begin{array}{*{20}{c}} {{H_2}O(l)} \\ 0 \\ {0.171\;mol} \\ {} \end{array}{/tex}
Applying Law of Chemical equilibrium
{tex}{K_c} = \frac{{[C{H_3}COO{C_2}{H_3}](l)[{H_2}O(l)]}}{{[C{H_3}COOH](l)[{C_2}{H_3}OH(l)]}}{/tex}
{tex} = \frac{{(0.171\;mol) \times (0.171\;mol)}}{{(0.829\;mol)(0.009\;mol)}} = 3.92{/tex}
Therefore, the equilibrium constant is 3.92. - {tex}\begin{array}{*{20}{c}} {}&{C{H_3}COOH(l) + }&{{C_2}{H_5}OH(l) \rightleftharpoons }&{C{H_3}COO{C_2}{H_5}(l)} \\ {Initial\;molar\;conc.}&{1.0\;mol}&{0.5\;mol}&{0.214} \\ {Molar\;conc.\;at}&{1.0 – 0.214}&{0.5 – 0.214}&{} \\ {equilibrium}&{ = 0.786}&{ = 0.286\;mol}&{} \end{array}\begin{array}{*{20}{c}} { + {H_2}O(l)} \\ {0.214mol} \\ {} \\ {} \end{array}{/tex}
{tex}{Q_c} = \frac{{[C{H_3}COO{C_2}{H_5}(l)][{H_2}O(l)]}}{{[C{H_3}COOH(l)][{C_2}{H_2}OH(l)]}}{/tex}
{tex} = \frac{{(0.214mol) \times (0.214\;mol)}}{{(0.286\;mol)(0.786\;mol)}} = 0.204{/tex}
Since Qc value 0.204 is less than Kc, value 3.92 this means that the equilibrium has not been reached. The reactants are still taking part in the reaction to form the products.
- The concentration ratio (concentration quotient) Qc for the reaction is:
- Answer the following:
- Structure:
{tex}\mathop {{\text{C}}{{\text{H}}_3}}\limits^1 – \mathop {\mathop {\mathop {{\text{CH}}}\limits^2 }\limits_| }\limits_{{\text{Br}}} – \mathop {\mathop {\mathop {\text{C}}\limits_{||} }\limits_{\text{O}} }\limits^3 – \mathop {\mathop {\mathop {{\text{CH}}}\limits_| }\limits_{{\text{C}}{{\text{H}}_{\text{3}}}} }\limits^4 – \mathop {{\text{C}}{{\text{H}}_3}}\limits^5 {/tex}
IUPAC name: 2-bromo-4-methyl-pentan-3-one. - % of P ={tex}\frac{62}{222} \times \frac{\text { weight of } \mathrm{Mg}_{2} \mathrm{P}_{2} \mathrm{O}_{7} \text { formed } \times 100}{\text { weight of organic compound }}{/tex}
= {tex}\frac{62}{222} \times \frac{0.22}{0.12} \times{/tex}100
= 51.20%
OR- Resonating structures of CH3COOH are:
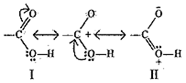
Resonating structures of CH3COO– are:
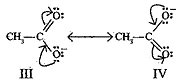
Structure (III) and (IV) are of equal energy and also contribute equally towards resonance hybrid of compound CH3COO–.
Structure II is less stable than Structure I because in structure II separation of +ve and-ve charges exists. Therefore, the contribution of structure (II) is less than that of structure (I) towards resonance hybrid of compound CH3COOH.
Therefore, CH3COO– is more stable than CH3COOH. - Carbon tetrachloride contains chlorine but it is bonded to carbon by covalent bond. Hence, it is not in ionic form.
So, it does not combine with AgNO3 solution.
Therefore, CCl4 does not give white precipitate with silver nitrate solution.
CCl4 + AgNO3 →No reaction.
- Structure:
Marking Scheme for the Class 11 exam
| Subject | Board Marks | Practical or internal Marks |
|---|---|---|
| English | 80 Marks | 20 Marks |
| Hindi | 80 Marks | 20 Marks |
| Mathematics | 80 Marks | 20 Marks |
| Chemistry | 70 Marks | 30 Marks |
| Physics | 70 Marks | 30 Marks |
| Biology | 70 Marks | 30 Marks |
| Computer Science | 70 Marks | 30 Marks |
| Informatics Practices | 70 Marks | 30 Marks |
| Chemistry | 80 Marks | 20 Marks |
| Business Studies | 80 Marks | 30 Marks |
| Economics | 80 Marks | 20 Marks |
| History | 80 Marks | 20 Marks |
| Political Science | 80 Marks | 20 Marks |
| Geography | 70 Marks | 30 Marks |
| Sociology | 80 Marks | 20 Marks |
| Physical Education | 70 Marks | 30 Marks |
| Home Science | 70 Marks | 30 Marks |
CBSE Sample Papers for Class 11
- Physics
- Chemistry
- Mathematics
- Biology
- Accountancy
- Economics
- Business Studies
- Computer Science
- Informatics Practices
- English Core
- Hindi Core
- Hindi Elective
- History
- Political Science
- Geography
- Sociology
- Physical Education
- Other Subjects
To download sample paper for class 11 Physics, Chemistry, Biology, History, Political Science, Economics, Geography, Computer Science, Home Science, Accountancy, Business Studies and Home Science; do check myCBSEguide app or website. myCBSEguide provides sample papers with solutions, test papers for chapter-wise practice, NCERT solutions, NCERT Exemplar solutions, quick revision notes for ready reference, CBSE guess papers and CBSE important question papers. Sample Papers all are made available through the best app for CBSE students and the myCBSEguide website.

Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Create Now
myCBSEguide
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Install Now