
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Install NowModel question paper for CBSE class 10 Science has 36 questions. The class 10 Science Sample Paper 2021 with the complete solution is available in myCBSEguide App for free download.
Class 10 Science Sample Paper
CBSE has made some basic changes this year. If you are not aware of these changes, be ready to encounter big surprises in the board exams. We sincerely advise all the students to go through the model question papers issued by CBSE, New Delhi and download the myCBSEguide App to get more and more sample papers with solutions. Here are some noticeable changes in CBSE class 10 Science Sample Paper 2021:
- Case study based MCQs
- 33% internal choice
- Assertion & Reason type questions
- Creative questions
- More subjective questions
Case study based Questions
This year CBSE, New Delhi has introduced the case study questions in almost all major subjects. Class 10 Science Sample Paper 2021 have four such questions from Q. No. 17 to 20. But need not to worry about that as they all are MCQ based questions. You may say that these are rather scoring questions if you have done a little practice on such questions. We again suggest you go through the myCBSEguide App and download these model question papers. This will help you master these case study type problems.
Here is the class 10 Science Sample Paper 2021. To download the solution to this question paper please visit the myCBSEguide Mobile App.
CBSE Class 10 Science
Sample Paper 01 (2020-21)
Maximum Marks: 80
Time Allowed: 3 hours
General Instructions:
- The question paper comprises four sections A, B, C and D. There are 36 questions in the question paper. All questions are compulsory.
- Section–A – question no. 1 to 20 – all questions and parts thereof are of one mark each. These questions contain multiple-choice questions (MCQs), very short answer questions and assertion – reason type questions. Answers to these should be given in one word or one sentence.
- Section–B – question no. 21 to 26 are short answer type questions, carrying 2 marks each. Answers to these questions should in the range of 30 to 50 words.
- Section–C – question no. 27 to 33 are short answer type questions, carrying 3 marks each. Answers to these questions should in the range of 50 to 80 words.
- Section–D – question no. 34 to 36 are long answer type questions carrying 5 marks each. Answers to these questions should be in the range of 80 to 120 words.
- There is no overall choice. However, internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
- Wherever necessary, neat and properly labeled diagrams should be drawn.
Section A
- Translate the following statements into chemical equations and then balance them:
Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.ORMethane burns in oxygen to form carbon dioxide and water. Write a balanced equation for the reaction. - Identify the reducing agent in the reaction:
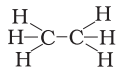
4NH3 + 5O2 ⟶⟶ 4NO + 6H2O - Structural formula of ethyne is
- H – C ≡≡ C – H
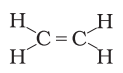
- H3 – C ≡≡ C – H
- Define the refractive index of a transparent medium. What is its unit? Which has a higher refractive index glass or water?
- Which phenomenon is responsible for increasing the apparent length of the day by 4 min?
- Give one use of bleaching powder.ORWhat are acids ?
- How many joule are there in 1 kWh?
- Identify the poles of a magnet in the figure.
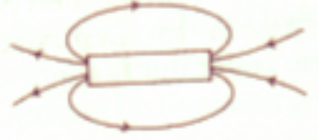
- A current of 1A is drawn by a filament of an electric bulb. What would be the number of electrons passing through a cross-section of the filament in 16s?ORWhen two ends of a metallic wire are connected across the terminals of a cell, then some potential difference is set up between its ends. In which direction electrons are flowing through the conductors?
- The process by which carbohydrates are synthesized by plants from carbon dioxide and water with help of chlorophyll and light.
- Name the components which you will observe when you focus the stomata slide under high power objective of a microscope.ORWrite one function of valves in the walls of veins.
- What are two main components of ecosystem?ORWhat are the various steps of food chains through which the transfer of food energy takes place is called?
- Chemosynthetic mode of nutrition is different from the photosynthetic mode in which way?
- Assertion: Photosynthesis is considered as an endothermic reaction.
Reason: Energy gets released in the process of photosynthesis.- Both assertion and reason are CORRECT and reason is the CORRECT explanation of the assertion.
- Both assertion and reason are CORRECT but, reason is NOT THE CORRECT explanation of the assertion.
- Assertion is CORRECT but, reason is INCORRECT.
- Assertion is INCORRECT but, reason is CORRECT.
- Assertion (A): Man is an omnivore.
Reason (R): Man eats food products obtained from both plants and animals.ORAssertion (A): Bile is essential for the digestion of lipids.
Reason (R) : Bile juice contains enzymes.- Both A and R are true and R is correct explanation of the assertion.
- Both A and R are true but R is not the correct explanation of of the assertion
- A is true but R is false.
- A is false but R is true.
- Both A and R are true and R is correct explanation of the assertion.
- Both A and R are true but R is not the correct explanation of the assertion
- A is true but R is false.
- A is false but R is true.
- Assertion (A): Variations are seen in offspring produced by asexual reproduction.
Reason (R): DNA molecules generated by replication is not exactly identical to original DNA.- Both A and R are true and R is correct explanation of the assertion.
- Both A and R are true but R is not the correct explanation of the assertion.
- A is false but R is true.
- A is true but R is false.
- Read the following and answer any four questions:
Sanjay studied about blood circulation in humans. He wanted to observe the flow of blood and was about to cut his finger a bit. He suddenly realized that this could be fatal.- What is the correct route for blood flow in a human?
- left auricle →→ left ventricle →→ lungs →→ right ventricle →→ right auricle
- left auricle →→ left ventricle →→ right ventricle →→ right auricle →→ lungs
- right auricle →→ right ventricle →→ left ventricle →→ left auricle →→ lungs
- right auricle →→ right ventricle →→ lungs →→ left auricle →→ left ventricle
- The diagram shows the vertical section through the heart:

What are the functions of the numbered blood vessels?carries blood to body carries blood to lungs carries blood from lungs carries blood from body (a) 1 2 3 4 (b) 1 3 4 2 (c) 2 4 3 1 (d) 3 1 4 2 - The table shows the characteristics of blood in one blood vessel of the body.
oxygen concentration carbon dioxide concentration pressure high low high Which blood vessel contains blood with these characteristics?
- Aorta
- Pulmonary artery
- Pulmonary vein
- Vena cava
- The colour of blood is red due to the presence of
- Hemocyanin
- Haemoglobin
- Chlorophyll
- Xanthophyll
- Which of the following chambers of the human heart contain oxygenated blood?
- Left auricle and left ventricle
- Left auricle and right ventricle
- Right auricle and left ventricle
- Right auricle and right ventricle
- What is the correct route for blood flow in a human?
- Read the following and answer any four questions:
Salt of a strong acid and strong base is neutral with a pH value of 7. NaCl common salt is formed by a combination of hydrochloride and sodium hydroxide solution. This is the salt that is used in food. Some salt is called rock salts bed of rack salt were formed when seas of bygone ages dried up. The common salt thus obtained is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, bleaching powder.- Which of the following does not form an acidic salt?
- Phosphoric acid
- Carbonic acid
- Hydrochloric acid
- Sulphuric acid
- Which of the following salts has no water of crystallization?
- Blue vitriol
- Washing soda
- Baking soda
- Gypsum
- The formula of baking soda is
- K2CO
- KHCO3
- NaHCO3
- Na2CO3
- Which of the following is treated with chlorine to obtain bleaching powder
- CaSO4
- Ca(OH)2
- Mg(OH)2
- KOH
- Which of the following salt is used for removing the permanent hardness of water
- Washing soda
- Baking soda
- Bleaching powder
- NaOH
- Which of the following does not form an acidic salt?
- Read the following and answer any four questions:
In resistance for a system of the resistor, there are two methods of joining the resistors together as shown below
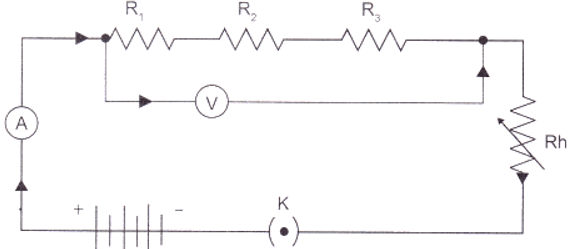
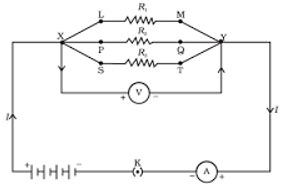
It showed an electric current in which 3 resistors having resistor R1, R2 and R3 respectively are join end to end i.e series. While the combination of the resistor in which 3 resistors connected together which point X and Y are said to be parallel.- The total potential difference across a combination of a resistor in series is equal to
- V1 + V2 + V3
- V1 – V2 +V2
- V1 + V2
- None of these
- In a series combination of resistor, the current is
- same at every point of the circuit
- different at every point of the circuit
- zero
- can not be determined
- The electrical energy disputed in the resistor is given by
- W = VIT
- W = VIR
- W = RIT
- W = RT
- If 5 resistor, each of value 0.2 ohm are connected in series what will be the resultant resistance
- 1 ohm
- 10 ohm
- 6 ohm
- 8 ohm
- The figure given below shows three resistors.
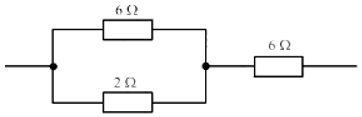
Their combined resistance is:- 16 ΩΩ
- 14 ΩΩ
- 203Ω203Ω
- 152Ω152Ω
- The total potential difference across a combination of a resistor in series is equal to
- Read the following and answer any four questions:
In the electrolytic refining of copper. The electrolyte is a solution of acidified copper sulphate. There are an anode and cathode. Refining is carried out by passing an electric current.
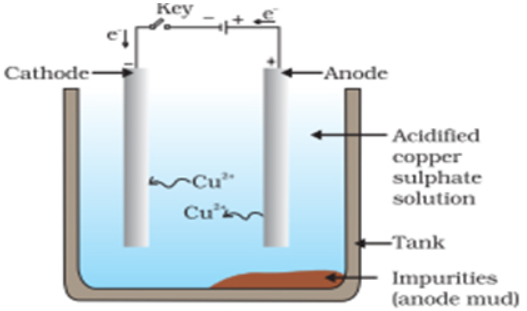
- The anode is ________.
- pure strips
- impure copper
- refined copper
- none of these
- Anode mud consists of:
- insoluble impurities
- soluble impurities
- pure metal
- impure metal
- Which of the following are refined electrolytically: (A) Au, (B) Cu, (C) Zn, (D) K
- A and B
- B and C
- A, B and C
- B, C and D
- On passing, electric current Cu is deposited on:
- cathode
- anode
- bottom of cathode
- bottom of anode
- Which one of the following four metal would be displaced from the solution of its salt by the other three metal?
- Zn
- Mg
- Cu
- Ag
- The anode is ________.
Section B
- What will happen if there is sudden shortage of water in the body? ORWhat is the difference between arteries & veins?
- State the two vital functions of kidney.
- What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur?
- With the help of a chemical equation, explain how a soda-acid fire extinguisher helps in putting out a fire.
- A ray passing through the centre of curvature of a spherical mirror after reflection, returns along the same path. Why?
- What is an electric fuse? What is its role in electric circuits? Should it be placed on neutral wire or on live wire? Justify your answer.
- Study the following cross that shows the self-pollination in F1, fill in the blank the genotype and phenotype in the F1 generation. What type of cross it is?
Parents RRYY x rryy Round, yellow wrinkled, green F1 — Rr Yy x ? Round, yellow OR
Distinguish between acquired and inherited characters with an example of each.
- Give an account of factors affecting the rate of decomposition.
- What are differences between aerobic and anaerobic respiration? Name some organisms that use anaerobic mode of respiration.
- A silver article generally turns black when kept in the open for a few days. The article when rubbed with toothpaste again starts shinning. Why do silver articles turn black when kept in the open for a few days? Name the phenomenon involved.
- What are the uses of Modern Periodic Table?
- The following table shows the positions of four elements A, B, C and D in the periodic table.
Group →→ 1 2 3 to 12 13 14 15 16 17 Period ↓↓ 2 A B 3 C D Using the above table answer the following questions.
- Which element will form only covalent compounds?
- Which element is a metal with valency 2?
- Out of C and D which one has bigger atomic radius and why?
- What is dispersion? What happens when light is passed through a glass prism?
- A thin converging lens form a real magnified image and virtual magnified image of an object in front of it.ORA 2.0 cm tall object is placed perpendicular to the principal axis of a convex lens of focal length 10 cm. The distance of the object from the lens is 15 cm. Find the nature, position, and size of the image. Also, find its magnification.
- Write the positions of the objects in each case.
- Draw ray diagrams to show the image formation in each case.
- How will the following be affected on cutting this lens into two halves along the principal axis?
- Focal length
- Intensity of the image formed by half lens.
- Reproduction is essentially a phenomenon that is not for survival of an individual but for the stability of a species. Justify.
- What is the pattern of magnetic field pattern due to current carrying conductor. OR
- Draw magnetic field lines produced around a current-carrying straight conductor passing through cardboard. Name, state and apply the rule to mark the direction of these field lines.
- How will the strength of the magnetic field change when the point where the magnetic field is to be determined is moved away from the straight wire carrying constant current? Justify your answer.

Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Create Now
myCBSEguide
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Install Now
And question no 19
Read More >>>> Class 10 Science MCQ <<<<<<