
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Install NowNCERT Solutions for Class 11 Chemistry Structure of Atom Part 4 Class 11 Chemistry book solutions are available in PDF format for free download. These ncert book chapter wise questions and answers are very helpful for CBSE board exam. CBSE recommends NCERT books and most of the questions in CBSE exam are asked from NCERT text books. Class 11 Chemistry chapter wise NCERT solution for Class 11 Chemistry Some Basic Concepts of Chemistry Part 1 and all the chapters can be downloaded from our website and myCBSEguide mobile app for free.
NCERT solutions for Class 11 Chemistry Download as PDF
NCERT Class 11 Chemistry Chapter wise Solutions
- Chapter 1 – Some Basic Concepts of Chemistry
- Chapter 2 – Structure of Atom
- Chapter 3 – Classification of Elements and Periodicity in Properties
- Chapter 4 – Chemical Bonding and Molecular Structure
- Chapter 5 – States of Matter
- Chapter 6 – Thermodynamics
- Chapter 7 – Equilibrium
- Chapter 8 – Redox Reactions
- Chapter 9 – Hydrogen
- Chapter 10 – The s-Block Elements
- Chapter 11 – The p-Block Elements
- Chapter 12 – Organic Chemistry – Some Basic Principles and Techniques
- Chapter 13 – Hydrocarbons
- Chapter 14 – Environmental Chemistry
NCERT Solutions for Class 11 Chemistry Structure of Atom Part 4
49. Lifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nano second range. If the radiation source has the duration of 2 ns and the number of photons emitted during the pulse source is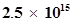 , calculate the energy of the source.
, calculate the energy of the source.
50. The longest wavelength doublet absorption transition is observed at 589 and 589.6 nm. Calculate the frequency of each transition and energy difference between two excited states.
51. The work function for caesium atom is 1.9 eV. Calculate (a) the threshold wavelength and (b) the threshold frequency of the radiation. If the caesium element is irradiated with a wavelength 500 nm, calculate the kinetic energy and the velocity of the ejected photoelectron.
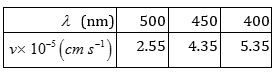
52. Following results are observed when sodium metal is irradiated with different wavelengths. Calculate (a) threshold wavelength and, (b) Planck’s constant.
53. The ejection of the photoelectron from the silver metal in the photoelectric effect experiment can be stopped by applying the voltage of 0.35 V when the radiation 256.7 nm is used. Calculate the work function for silver metal.
54. If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of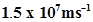 , calculate the energy with which it is bound to the nucleus.
, calculate the energy with which it is bound to the nucleus.
55. Emission transitions in the Paschen series end at orbit n= 3 and start from orbit n and can be represented as 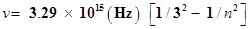
Calculate the value of n if the transition is observed at 1285 nm. Find the region of the spectrum.
56. Calculate the wavelength for the emission transition if it starts from the orbit having radius 1.3225 nm and ends at 211.6 pm. Name the series to which this transition belongs and the region of the spectrum.
57. Dual behaviour of matter proposed by de Broglie led to the discovery of electron microscope often used for the highly magnified images of biological molecules and other type of material. If the velocity of the electron in this microscope is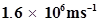 , calculate de Broglie wavelength associated with this electron.
, calculate de Broglie wavelength associated with this electron.
58. Similar to electron diffraction, neutron diffraction microscope is also used for the determination of the structure of molecules. If the wavelength used here is 800 pm, calculate the characteristic velocity associated with the neutron.
59. If the velocity of the electron in Bohr’s first orbit is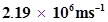 , calculate the de Broglie wavelength associated with it.
, calculate the de Broglie wavelength associated with it.
60. The velocity associated with a proton moving in a potential difference of 1000 V is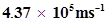 . If the hockey ball of mass 0.1 kg is moving with this velocity, calculate the wavelength associated with this velocity.
. If the hockey ball of mass 0.1 kg is moving with this velocity, calculate the wavelength associated with this velocity.
61. If the position of the electron is measured within an accuracy of + 0.002 nm, calculate the uncertainty in the momentum of the electron. Suppose the momentum of the electron is 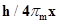 =0.05 nm, is there any problem in defining this value.
=0.05 nm, is there any problem in defining this value.
62. The quantum numbers of six electrons are given below. Arrange them in order of increasing energies. If any of these combination(s) has/have the same energy lists:
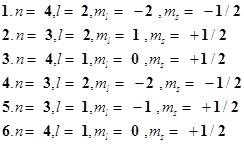
63. The bromine atom possesses 35 electrons. It contains 6 electrons in 2 porbital, 6 electrons in 3porbital and 5 electrons in 4porbital. Which of these electron experiences the lowest effective nuclear charge?
64. Among the following pairs of orbitals which orbital will experience the larger effective nuclear charge? (i) 2s and 3s, (ii) 4d and 4f, (iii) 3d and 3p
65. The unpaired electrons in Al and Si are present in 3p orbital. Which electrons will experience more effective nuclear charge from the nucleus?
66. Indicate the number of unpaired electrons in: (a) P, (b) Si, (c) Cr, (d) Fe and (e) Kr.
67. (a) How many sub-shells are associated with n= 4? (b) How many electrons will be present in the sub-shells having 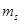 value of
value of 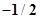 for n = 4?
for n = 4?
NCERT Solutions for Class 11 Chemistry Chapter 2 Structure of Atom Part 4
NCERT Solutions Class 11 Chemistry Structure of Atom Part 4 PDF (Download) Free from myCBSEguide app and myCBSEguide website. Ncert solution class 11 Chemistry includes text book solutions from both part 1 and part 2. NCERT Solutions for CBSE Class 11 Chemistry have total 14 chapters. 11 Chemistry NCERT Solutions in PDF for free Download on our website. Ncert Chemistry class 11 solutions PDF and Chemistry ncert class 11 PDF solutions with latest modifications and as per the latest CBSE syllabus are only available in myCBSEguide
NCERT Solutions for Science Class 3rd to 12th
- NCERT Solutions for Class 12 Physics.
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for Class 12 Biology
- NCERT solutions for class 11 Physics
- NCERT Solutions for Class 11 Chemistry
- NCERT solutions for class 11 Biology
- NCERT Solutions for Class 10 Science
- NCERT Solutions for Class 9 Science
- NCERT solutions for Class 8 Science
- NCERT solutions for Class 7 Science
- NCERT Solutions for Class 6 Science
- NCERT Solutions for Class 5 EVS
- NCERT Solutions for Class 4 EVS
- NCERT Solutions for Class 3 EVS
To download NCERT Solutions for class 11 Chemistry, Physics, Biology, History, Political Science, Economics, Geography, Computer Science, Home Science, Accountancy, Business Studies and Home Science; do check myCBSEguide app or website. myCBSEguide provides sample papers with solution, test papers for chapter-wise practice, NCERT solutions, NCERT Exemplar solutions, quick revision notes for ready reference, CBSE guess papers and CBSE important question papers. Sample Paper all are made available through the best app for CBSE students and myCBSEguide.com website.

Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Create Now
myCBSEguide
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Install Now